— Results to date demonstrate rapid post-surgical visual recovery with no serious adverse events and meet or exceed FDA target rates for safety and effectiveness –
— Data presented today at Eyecelerator meeting in Park City —
CAMBRIDGE, Mass., May 2, 2025 /PRNewswire/ — Pykus Therapeutics, Inc. (“Pykus” or the “Company”) today announced the presentation of positive clinical data from of its ongoing pilot study evaluating the use of its proprietary, lead product candidate, PYK-2101, a focal hydrogel retinal sealant, in patients undergoing surgery for detached retina. The retinal attachment rate in this trial exceeded the FDA target rate for ocular endotamponades; potential for rapid vision recovery was demonstrated; and no safety concerns were raised. Interim results from PYK-2101-RD001 were presented by James (Tony) Stefater, MD, PhD, President and Pykus cofounder at Eyecelerator @ Park City on May 2nd, 2025.
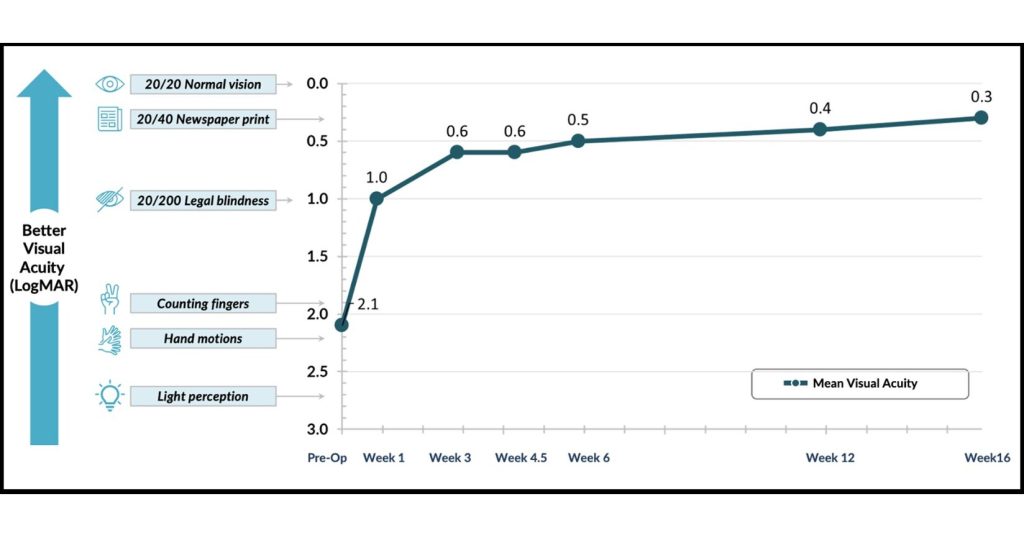
Figure 1. PYK-2101 demonstrates rapid vision recovery following surgery
Please contact the Company directly for more information regarding the Eyecelerator presentation.
“PYK-2101 has the potential to offer enormous benefits to patients undergoing retinal detachment surgery,” said Dean Eliott, MD, Stelios Evangelos Gragoudas Professor of Ophthalmology at Harvard Medical School at Massachusetts Eye and Ear Infirmary, and Director of the Retina Fellowship at Harvard/Mass. Eye and Ear. “Following surgery, patients currently must remain in a prone, ‘face-down’ position almost 24 hours per day, must contend with work and travel restrictions, and have poor vision for weeks following surgery. By improving vision and eliminating ‘face-down’ positioning, this product could offer a transformative improvement in retinal detachment surgery.”
“We are thrilled with the results shown to date from our pilot clinical trial, and look forward to sharing additional data upon completion of our trial in the coming months,” said Dr. Stefater.
PYK-2101-RD001 is a prospective, multicenter, open-label pilot trial examining the use of PYK-2101 in 11 patients with retinal detachment undergoing pars plana vitrectomy. The objective of this study is to evaluate the safety and tolerability of PYK-2101 within the first 16 weeks post vitrectomy. Outcome measures include anatomical attachment rate, speed of visual acuity recovery, degree of cataract progression, change in intraocular pressure, and adverse events. The study is being conducted at leading vitreoretinal clinics in Australia.
Results to date include the following highlights:
- No serious AEs or SAEs, and no direct effects of PYK-2101 on IOP were observed.
- PYK-2101 has demonstrated a single surgery retinal attachment rate of 91% in the per protocol (PP) population1 and 73% in the intent to treat (ITT) population1 vs. an FDA target rate of 72%2.
- PYK-2101 demonstrates potential for rapid vision recovery (Figure 1).
(1) Reflects subject data as of April 21, 2025 (2) FDA target rate sourced from Alcon’s C3F8 PMA Summary of Safety and Effectiveness.
About PYK-2101:
PYK-2101 is a patented, first-in-class biodegradable retinal hydrogel sealant. The Company is pursuing an initial indication for the treatment of retinal detachment. PYK-2101 offers the opportunity to dramatically improve and accelerate visual recovery following retinal detachment surgery by sealing retinal breaks directly without having to obscure vision, while eliminating the need for patients to position “face-down” after surgery. Compared to the current standard-of-care, which involves filling the eye with endotamponade agents such as intraocular gases or silicone oil, PYK-2101 aims to dramatically enhance the patient experience and improve surgical outcomes.
About Retinal Detachment Surgery and Vitrectomy Surgery:
Nearly two million retinal surgeries, or vitrectomies, are performed annually worldwide. Retinal Detachment is amongst the most common indications for undergoing retinal surgery. The current standard-of-care for retinal detachment surgery exposes patients to significant post-operative burdens, prolonged visual recovery, and fails in a high proportion of cases, requiring repeat surgery resulting in permanent vision loss.
About Pykus Therapeutics:
Pykus Therapeutics, Inc., based in Cambridge, MA, is a clinical-stage, medical technology company dedicated to advancing treatments for retinal and other ophthalmic diseases. Using technology originally developed by and licensed from Mass Eye and Ear (now part of Mass General Brigham) at Harvard Medical School, Pykus aims to deliver transformative solutions to improve surgical outcomes and enhance patient care. For more information, visit www.pykustherapeutics.com.
Contact:
Chris White
Pykus Therapeutics
[email protected]
SOURCE Pykus Therapeutics